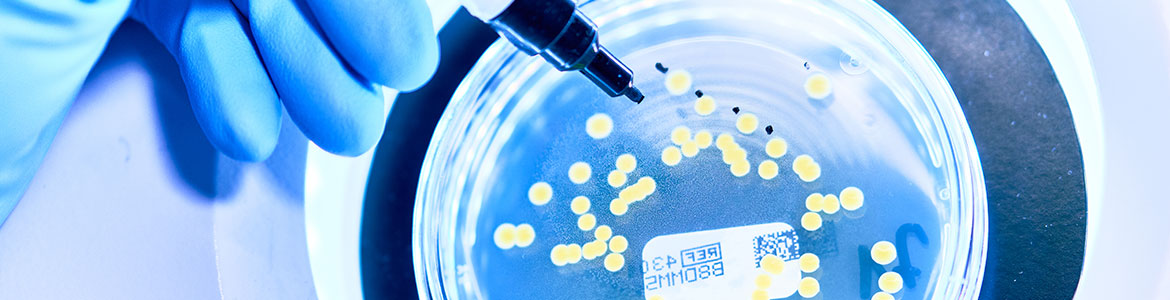
Bioburden determination
Numerous medical devices, single-use devices or primary packaging materials in the pharmaceutical industry are subjected to sterilisation due to their intended use. For the sterilisation process to be successful, only a certain number of germs may be present on the surface of the product or the packaging. The term bioburden refers to the number of germs on the surface of a product prior to sterilisation.
Testing for bioburden is regulated in DIN ISO 11737-1 is binding for the manufacturers of medical devices.
Related Topics: