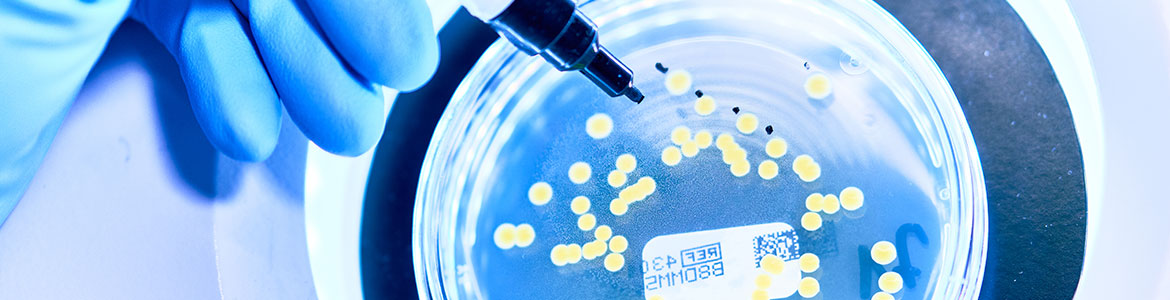
Total microbial count determination
For the microbiological quality and safety of a product, the number of viable microorganisms such as bacteria and fungi that a product contains is decisive. The total germ count is determined and this test is an important part of ensuring product quality. Testing is performed in accordance with pharmacopoeia regulations, standardised procedures, customer-specific test procedures or DIN EN ISO standards.
The suitability test of the method and method transfers are performed in coordination with our customers within the framework of accreditation according to DIN EN ISO 17025 or under GMP. Labor LS offers the following services in the field of total microbial count testing:
- Quantitative testing: determining the total microbial count in the product (number of viable microorganisms such as bacteria and fungi)
- Qualitative testing: detection of specified microorganisms in the product (e.g. salmonella, Pseudomonas aeruginosa and Staphylococcus aureus)
- Testing in accordance with the requirements of the valid pharmacopoeias (Ph. Eur., USP, JP) and other international pharmacopoeias (e.g. CP, Ph. Rus.),
- Testing within the framework of standardised procedures such as DIN EN ISO 11737-1 for medical devices and packaging materials
- Testing within the framework of DIN standards for cosmetic products
- Preparation of risk assessments by Labor LS
- Total microbial count of high-purity water, pure steam and process water (AP water and WFI) and raw materials
- Total microbial count within the context of in-process controls